The structure of the water
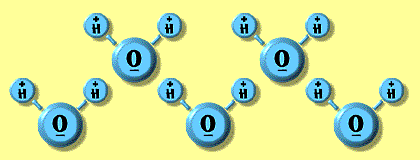
| All the observable behaviors of water can be explained by examining the structure and composition of water molecules themselves. As you know, water molecules are composed of two atoms of hydrogen and one atom of oxygen. Because of the different characteristics of the oxygen and hydrogen atoms, the area of the water molecule near the oxygen atom is slightly negatively charged and the area near the hydrogen atoms is slightly positively charged. Molecules that have this type of charge separation are called polar molecules. It is this polar property of water molecules that gives water many of its unusual characteristics. The polar nature of a water molecule can be illustrated like showed in the right. |

|
|
The polar nature of water causes water molecules to attract each other between their positive and negative ends. This mutual attraction can be illustrated like this:
A substance such as wax is non-polar and has few molecules with a net charge or charge separation. Therefore, water molecules do not adhere well to wax paper. This is why the water beads up and moves along the way it does on wax paper in Racedrop Raceway. |